Li Li, Assistant Professor of Biomedical Sciences, UMass Chan Medical School
Vaccines have been reliably and affordably protecting people from diseases worldwide for centuries. Until the COVID-19 pandemic, however, vaccine development was still a long and idiosyncratic process. Traditionally, researchers had to tailor manufacturing processes and facilities for each vaccine candidate, and the scientific knowledge gained from one vaccine was often not directly transferable to another.
But the COVID-19 mRNA vaccines brought a new approach to vaccine development that has far-reaching implications for how researchers make drugs to treat many other diseases.
I am a biochemist, and my lab at UMass Chan Medical School focuses on developing better ways to use mRNA as a drug. Although there are many possibilities for what researchers can use mRNA to treat, some important limitations remain. Better understanding how mRNA-based drugs interact with the immune system and how they are degraded in human cells can help lead to safe, durable and effective treatments for a wide range of diseases.
Some basics of mRNA drugs
Messenger RNA, or mRNA, is made of four building blocks denoted by the letters A, C, G and U. The sequence of letters in an mRNA molecule conveys genetic information that directs how a protein is made.
Get facts about the coronavirus pandemic and the latest research
Science newsletter
An mRNA drug comprises two essential components: mRNA molecules, which code for desired proteins, and the lipid molecules – such as phospholipids and cholesterol – that encapsulate them. These mRNA-lipid nanoparticles, or LNPs, are tiny spheres about 100 nanometers in diameter that protect mRNA from degradation and facilitate its delivery into target cells.
Once inside cells, mRNA molecules instruct the cell’s machinery to produce the target protein required for a desired therapeutic effect. For example, the mRNA in the Pfizer-BioNTech and Moderna COVID-19 vaccines directs cells to produce a harmless version of the virus’ spike protein that trains the immune system to recognize and better prepare for potential infection.The science behind COVID-19 mRNA vaccines has been decades in the making.
From a drug development perspective, mRNA drugs offer significant advantages over traditional drugs because they are easily programmable. Hundreds of pounds of mRNA can be made from readily available DNA templates, such that producing a different mRNA drug is as simple as changing the corresponding DNA templates.
More importantly, different mRNA drugs produced by the same set of methods will have similar properties. They will be delivered to the same tissues, trigger similar levels of immune responses and degrade in similar ways. This predictability significantly reduces the development risks and financial costs of developing mRNA drugs.
In addition to being easy to program, mRNA drugs have several other unique properties. For example, just like the mRNAs your body naturally produces, therapeutic mRNAs have a short half-life in cells: about one day. As a result, current mRNA technology is ideal for treatments that aren’t meant to last long in the body.
This is why vaccines are popular candidates for mRNA technology: They provide long-term protection against disease after brief exposure to the drug with few side effects. There are currently more than 30 mRNA vaccine candidates, not including vaccines for COVID-19, in clinical trials.
Self vs. nonself
Another critical feature of mRNA drugs is their intrinsic ability to stimulate the immune system. This may sound paradoxical – after all, your cells already contain large amounts of mRNAs. Why would other mRNAs activate your immune system? How does your immune system distinguish between self and nonself mRNAs?
The first reason involves location. Therapeutic mRNAs enter cells using endosomes – sacs made of the cell’s membrane that take in materials from the cell’s environment. Your immune system can detect mRNA in endosomes because this is usually a sign of an RNA virus infection – cellular mRNAs normally don’t enter endosomes. When your immune system labels therapeutic mRNAs as viral material, it triggers a strong inflammatory response that can lead to severe side effects.
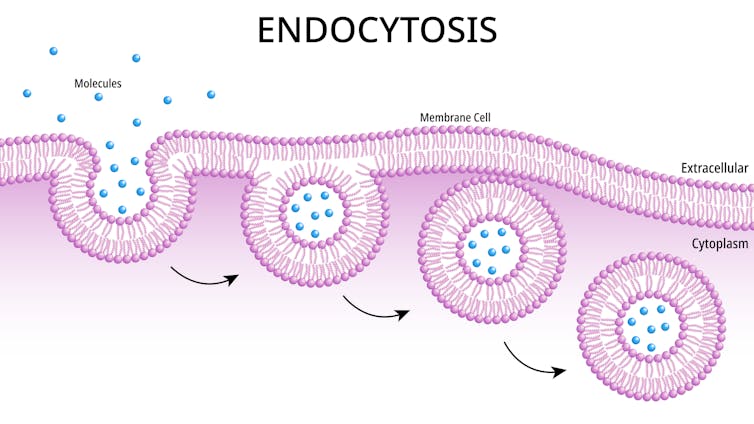
One solution to this problem is to modify mRNA’s building blocks – specifically, changing the U, or uridine, to its chemical cousins, pseudouridine and N1-methylpseudouridine. This subtle chemical change prevents the unwanted immune response while allowing the therapeutic mRNA to direct the cell to make the protein it encodes. The 2023 Nobel Prize in physiology or medicine was awarded to the scientists who made this breakthrough discovery. Both the Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines use this technique.
The second source of unwanted immune response is impurities from mRNA production. To prepare mRNA from a DNA template, scientists use a protein called RNA polymerase that tends to make a small amount of side product called double-stranded RNA. Unlike mRNA, which is single-stranded, double-stranded RNA has two chains that form a double helix. RNA viruses also form double-stranded RNA when they replicate, and exposing cells to double-stranded RNA can lead to a strong immune response.
Removing double-stranded RNA is challenging, especially at the industrial scale. Fortuitously, for mRNA vaccines, the residual amount of double-stranded RNA can stimulate the immune system to enhance antibody responses. However, for applications other than vaccines, a cleaner RNA product is necessary to reduce side effects.
Moving beyond vaccines
Although mRNA has the potential to transform drug development for various medical purposes, careful consideration is required to identify targets that align with the technology’s strengths.
For example, because there is currently a limit to how long mRNA can last in the body, treatments that need a protein to be present for only a short period of time to achieve a lasting therapeutic effect are ideal. One promising example in development is using mRNA that encodes CRISPR-Cas9 gene-editing proteins to knock out genes that cause specific diseases.
Researchers are exploring this strategy to develop a single-dose treatment for hereditary transthyretin amyloidosis, a rare genetic disease caused by the accumulation of misfolded proteins in the heart and nerves. This disease is an ideal target for mRNA-based CRISPR gene therapy because the target protein is produced by the liver. Because most drugs pass through the liver, this makes it easier to deliver CRISPR-Cas9 mRNA to its target. In the next few years, a new generation of more precise mRNA-based genome editing therapies will enter clinical trials.
For treatments that need a specific protein to be present in the body for long periods of time or need to prompt little to no immune reaction, further advancements in mRNA technology are necessary to extend mRNA’s half-life and eliminate immune-triggering contaminants. Notable new developments in these areas include using computational algorithms to optimize mRNA sequences in ways that enhance their stability and engineering RNA polymerases that introduce fewer side products that may cause an immune response.
Further advancements have the potential to enable a new generation of safe, durable and effective mRNA therapeutics for applications beyond vaccines.






