Jamie Rowen, UMass Amherst and Tami S. Rowen, University of California, San Francisco
On April 21, 2023, the U.S. Supreme Court ruled that the abortion pill mifepristone, which is used in more than half of all abortions in the U.S., could remain accessible without restrictions – at least for now. The decision is temporary, however, buying time as an appeals court weighs the challenge to mifepristone brought by a Texas judge in early April 2023.
That ruling blocked the use of the drug in medication abortions and sought to remove it from the market altogether, questioning its safety. Days later, a U.S. appeals court reversed the suspension on mifepristone but placed tighter restrictions on it, including preventing it from being sent through the mail.
The Conversation asked twin sisters Jamie Rowen, a legal scholar, and obstetrician and gynecologist Tami Rowen to put into perspective what the Supreme Court’s decision means for access to the drug moving forward and how it came under legal scrutiny to begin with.
1. What led up to the Supreme Court’s ruling on mifepristone?
In September 2022, several groups of anti-abortion doctors sued the Food and Drug Administration, arguing that they were harmed because the FDA’s 2000 approval of mifepristone was flawed and that it did not adequately test the drug for safety, among other claims. The plaintiffs also claimed harm from the FDA’s 2016 and 2021 changes that lifted several restrictions on how the drug could be used or administered.
The doctors brought the case in Texas, where a federal district judge ordered that, while the case was pending, mifepristone should be off the market.
The FDA appealed to the 5th Circuit, asking it for an emergency “stay,” or a hold on, the district court’s order. The 5th Circuit ordered that, while the case is being decided, mifepristone can be on the market but only with its original restrictions from 2000. Under this order, mifepristone could only be used up to seven weeks of pregnancy and required an in-person visit and prescription from a doctor.
The FDA, along with mifepristone’s manufacturer Danco Laboratories, immediately asked the Supreme Court to stay the 5th Circuit’s order. Supreme Court stays are granted when at least five justices agree that the applicants – in this case the FDA and Danco – are likely to succeed, among other considerations.
The majority did not explain its decision in favor of the FDA and Danco. The two dissents – from Samuel Alito and Clarence Thomas – provide little insight into how the different justices might rule on the case if they decide to review the 5th Circuit’s forthcoming opinion.
2. What comes next in the courts?
The Supreme Court’s decision means that mifepristone will remain available until there is a final decision in this case. For now, the case returns to the 5th Circuit. Depending on the outcome of that case, either the plaintiffs or the defendants may ask the Supreme Court to hear the case. If the Supreme Court decides to hear the case, then the final decision on whether mifepristone should be taken off the market or have stricter requirements for use will come from the Supreme Court. If not, the final decision will come from the 5th Circuit.
Although the 5th Circuit is scheduled to hear the case on May 17, 2023, there is no fixed time by which it must make its decision. In short, it will likely take at least a year for the case to be decided. Regardless of these lower court decisions, the fact that at least five justices chose to stay the 5th Circuit’s emergency order suggests that the Supreme Court will want to make the final determination in this case.
3. What does this mean for abortion access moving forward?
The Supreme Court’s decision to preserve full access to mifepristone until the case concludes leaves the FDA’s current rules in place. These rules allow mifepristone to be administered up to 10 weeks of pregnancy without an in-person visit to a clinic or hospital, through the mail and by a certified pharmacy as an alternative to a doctor’s prescription.
Given the legal uncertainty and the amount of time it takes for a case like this to conclude, the Supreme Court’s April 21, 2023, decision enables ongoing access to mifepristone for the foreseeable future. Roughly 90,000 medication abortions are performed annually in the U.S., the vast majority of which rely on mifepristone as part of a two-medication regimen that also includes the drug misoprostol.
Mifepristone blocks the hormone progesterone, which is needed for a pregnancy to continue. Misoprostol, which is approved for use in the treatment of gastric ulcers, also causes uterine contractions and ends the pregnancy.
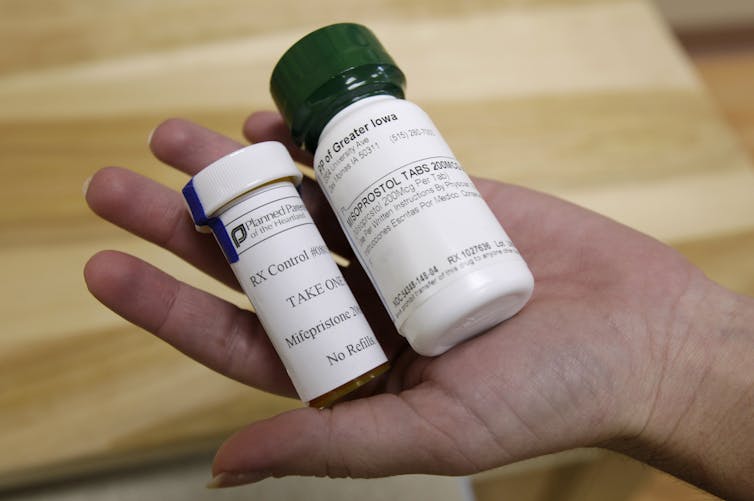
If the ultimate decision is in favor of the plaintiff doctors, the effects on pregnant people could be felt immediately. Taking mifepristone off the market until the FDA makes safety findings that are sufficient to the court, or restricting access to it through additional requirements, would lead people seeking medication abortions to use a misoprostol-only regimen or to seek surgical abortions. Though safe and effective, the misoprostol-only alternative would lead to higher rates of incomplete abortions that require additional, usually surgical, intervention. These procedures would exacerbate harms to those electing or experiencing abortion, including risks to subsequent pregnancies.
Likewise, forcing people to delay their abortions imposes numerous health risks. Even Supreme Court justices ambivalent about legal rights to abortion have expressed a desire for abortions to occur as early as possible.
Limiting access to mifepristone could have additional harmful effects. Mifepristone also helps women complete a miscarriage at a much higher success rate than the standard medical regimens that do not use mifepristone, sparing the risk of a surgical procedure and complications if the pregnancy remains in the uterus.
For now, the Supreme Court has created a buffer to help reduce such obstacles and adverse events while the lower courts, and likely the Supreme Court itself, decide the case.
4. What are the implications for other medications?
The Supreme Court did not explain whether it thinks the plaintiffs will be successful in their argument that the FDA should not have approved mifepristone in 2000 or changed the rules around its use in subsequent years.
When questioning an administrative agency, such as the FDA, a court asks whether the regulation was “arbitrary and capricious.” The 5th Circuit agreed with the district court that the 2016 regulation change was arbitrary and capricious because there was no study showing the effects of removing multiple restrictions on the medication at once. The FDA did review multiple studies that showed lifting these individual restrictions was indeed safe for those taking mifepristone.
Second-guessing the agency’s scientific determination in this way challenges the nuts and bolts of the FDA’s process and certainty in the drug manufacturing market. This is particularly true for medicine that may have higher risks but can be lifesaving for patients. Undermining the FDA’s authority could also carry over to controversial medications like the COVID-19 vaccine or even the vaccine against human papillomavirus, or HPV, the most common sexually transmitted infection in the U.S. Given parental concerns about vaccine safety and the belief that making sex medically safer for young people encourages them to have sex, the HPV vaccine has faced heightened scrutiny from vaccine opponents about its safety record.
Leaders from across the scientific, pharmacologic and business world have sounded the alarm at the implications of these decisions on approved drugs and those in development.
Finally, the legal wrangling over mifepristone will no doubt affect ongoing research into the many potential uses of this medication beyond abortion. These legal challenges delayed the introduction of mifepristone to the U.S. market decades ago, and they continue to impair studies on mifepristone’s potential to help prevent certain cancers, uterine infections and other illnesses affected by progesterone.
For now, the Supreme Court has put off a decision that could profoundly change the regulation of medicines in the U.S.
Jamie Rowen, Associate Professor of Legal Studies and Political Science, UMass Amherst and Tami S. Rowen, Associate Professor of Obstetrics, Gynecology and Gynecologic Surgery, University of California, San Francisco
This article is republished from The Conversation under a Creative Commons license. Read the original article.






